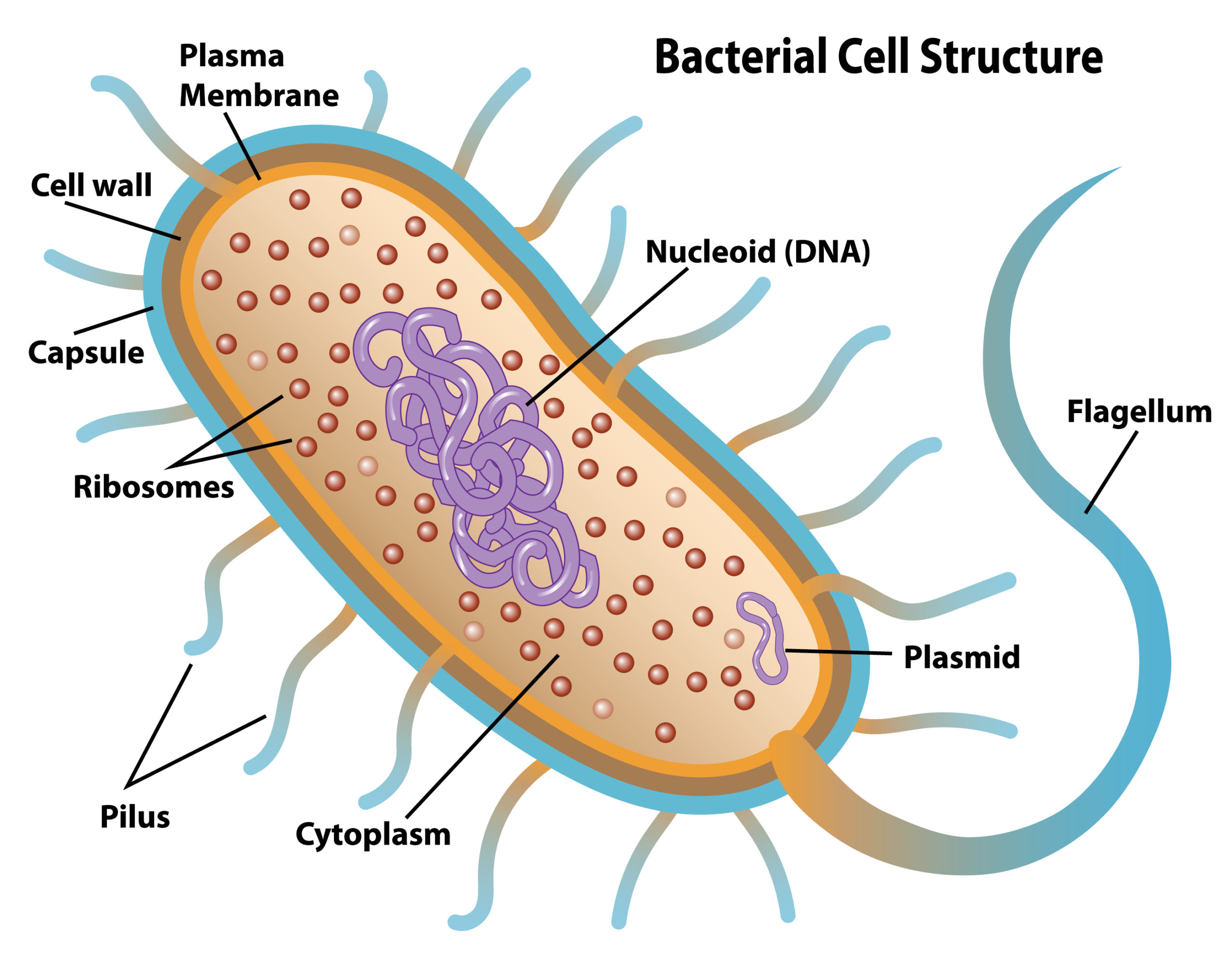
Some terminologies are not only complex but also uncommon to come by, and plasmids are just one of these terminologies. This article provides an A-Z of what readers should know about plasmids. But what are plasmids?
What is Plasmid?
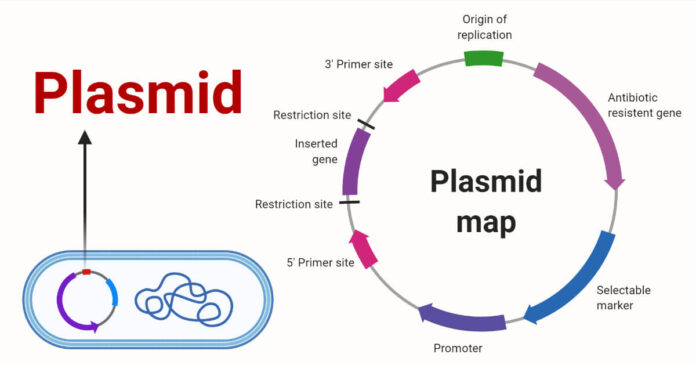
A plasmid is extrachromosomal DNA not incorporated into the main helix of genomic DNA. Plasmids occur in nature and can be found everywhere—in plants, insects, and bacteria. Scientists have discovered various applications for plasmids, including gene therapy and DNA vaccines.
Plasmid Delivery
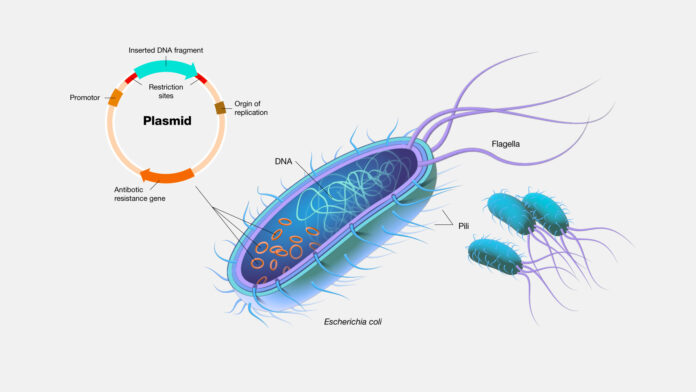
These applications deliver the plasmid to a specific target in an organism to obtain a desired response. Plasmids can be delivered as a naked molecule or encapsulated in a nanoparticle. Other delivery methods are being developed to improve plasmid delivery efficiency and increase serum survival lengths.
Fine Detail About Plasmids
Most plasmids are designed to contain an origin of replication, a gene of interest, and a gene for antibiotic resistance. In a process called cloning, a gene is inserted into an empty plasmid backbone (vector) using genetic engineering. Plasmids are specifically designed to replicate in bacteria without gene expression; yet, in their intended target, they express the gene without replication.
Once a plasmid is assembled with the proper components, it is transformed into a competent cell. In this process, electrical current or chemistry opens the bacterial pores to accept the plasmid, and the competent cells end up with several copies of the plasmid. To isolate only the plasmid-containing bacteria, the cells are cultured with the antibiotic corresponding to their antibiotic-resistant gene.
Since the plasmid includes a resistance marker to the antibiotic, a cell that accepts the plasmid will resist the antibiotic. Cells that reject the plasmid will not be resistant and will be instantly killed. The resistant cells are frozen as the master cell bank (MCB), and the MCB is used to make a working cell bank (WCB).
Upon completion of cell banking, the WCB is then fermented to replicate enough plasmid for use in animal studies and clinical trials.
Overall, readers who wonder what plasmids are should consider this piece and read more on the topic in this article.
Process Development of Plasmid
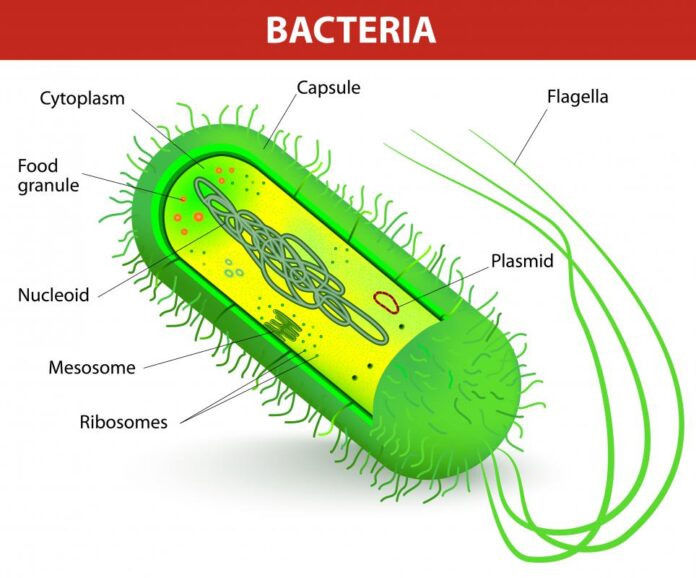
Every plasmid is different, and varying parameters such as plasmid size, stability, presence of secondary structures, ITR regions, etc., can affect yield and quality. It is critical for these factors to be considered during initial project discussions and the planning of the project scope.
Cell Bank Optimization
The process development lab can assess a variety of cell lines and screen several colonies to generate a high-quality cell bank. Evaluation of the selected colony by high density fermentation and purification allows the most accurate determination of cell bank performance before generating the GMP master cell bank and GMP plasmid production.
Fermentation Optimization
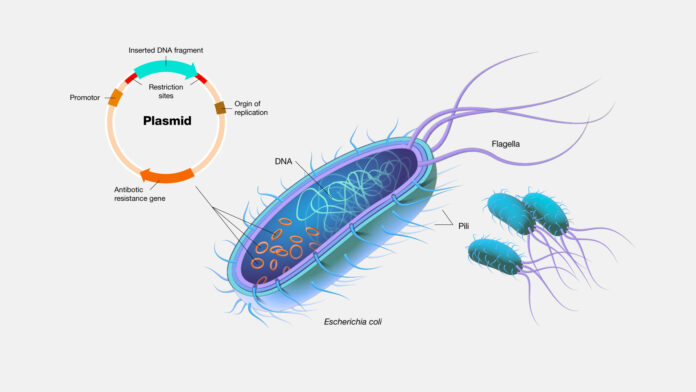
What are plasmids known for? For some plasmids, a series of small-scale (10 L) fermentation runs may be conducted under various growth conditions. Biomass, yield, and plasmid quality are assessed to maximize productivity and quality for downstream applications.
Purification Optimization
Small-scale purification studies are performed using methods representative of the full-scale purification process. Conditions are optimized based on plasmid size and performance at each purification step. Quality Control performs a series of stringent evaluation assays on the final product, which, once approved, is delivered to the customer along with a detailed certificate of analysis. It answers the question of what are plasmids.
GMP Cell Banking
Creating a high-quality, high-yielding master cell bank (MCB) provides a solid foundation for a successful GMP plasmid manufacturing campaign. The MCB is generated directly from a carefully screened and characterized research cell bank to ensure optimal performance in downstream manufacturing.
All cGMP cell banking activities are performed in a dedicated cGMP cell banking area. Manufacturing can utilize a client’s preferred bacterial strain, or a recommendation can be provided based on standard strains that have demonstrated high performance for plasmid DNA production.
GMP Fill/Finish
For applications requiring a final formulation and fill into vials, VGXI can provide convenience, in-house GMP fills/finish services. The QC-released bulk drug substance is adjusted to the specified concentration and, if applicable, formulated to contain any non-active or excipient components. The formulated drug product pool is then aseptically filtered and filled via an automated process within an ISO 5 filling enclosure.
Production for Clinical Commercial Supply
The cGMP Production Service provides injectable-grade plasmid DNA suitable for human clinical trials. The highly experienced team can work with the client’s unique project requirements to create a manufacturing solution that ensures the success of their clinical program.
Applications
- Human clinical trials
- Diagnostic reagents
- Clinical through the commercial supply of raw material for GMP viral vector and cell therapy production
Capabilities
- GMP E. coli master cell bank (MCB) production
- 40L to 500L fermentation
- The fully scalable purification process
- In-house GMP fill/finish capabilities for the production of final drug products
- Established partnership and proven technology transfer support for clients transitioning to fermentation/production scales greater than 500L
Recommended Testing for Plasmid Master Cell Banks

- Host Identification
- Identification of E. coli K12 strain
- Culture Purity
- Detection of Bacteriophage
- Cell Viability
- Plasmid Retention
- Plasmid Identification by DNA Sequencing
Recommended Testing for Plasmid Products
- Nucleic Acid Concentration
- Purity by A260/280
- Identity by AGE
- Analysis of Total Supercoil Forms by HPLC
- Analysis of Total Circular Forms by HPLC
- Host-Cell RNA
- Host-Cell Protein
- Host-Cell DNA
- Endotoxin
- pH
- Appearance
- Bioburden or Sterility
In addition to the recommended testing above, additional testing is available per client request.
Supporting Services
A range of additional services is available, including:
- Real-time and accelerated stability testing for bulk and filled products
- Process cost analysis
- Assay development
- Cell bank characterization
- Complete release testing for all plasmid products
- CMC support
- Validation services
- cGMP compliance audits and consulting
The pre-clinical production service employs the same patented manufacturing technology as our cGMP service and can be scaled to meet clinical demand without compromising quality.
Applications
- Small to large animal studies
- DNA vaccines
- Gene therapies
Capabilities
- 10L to 100L fermentation
- 100% of purified plasmid delivered
- Detailed certificate of analysis
- Fast turnaround and delivery