“Though HBOT hasn’t been proving clinically to cure or be highly effective at treating diabetes, autism or cancer, a quick internet research shows a range of claims and effectiveness for these conditions and others for which HBOT has not been approved by FDA.”
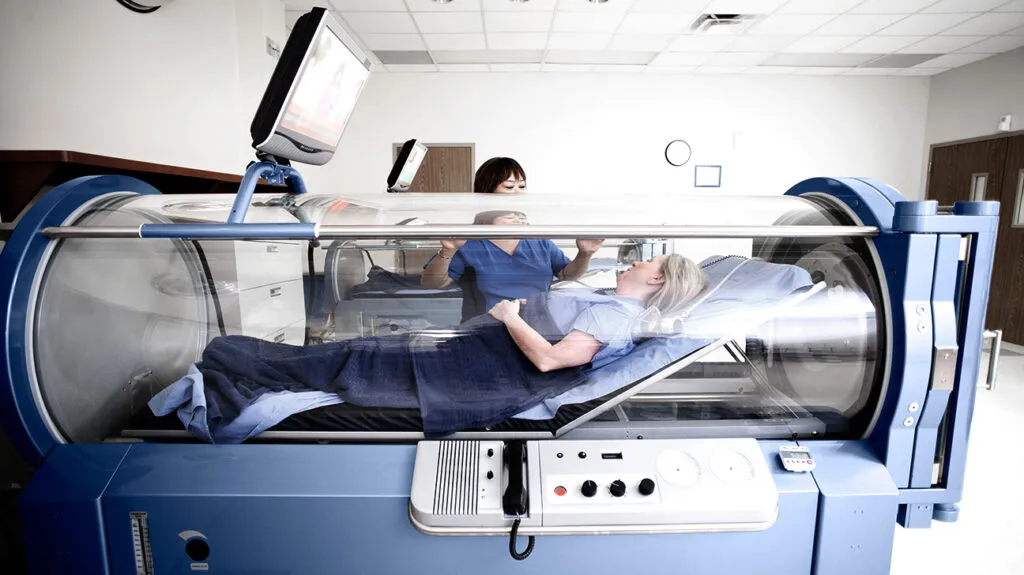
Hyperbaric oxygen therapy is a popular treatment for deep sea divers, and scuba divers affected by the abnormal change in temperature around them. But did you know that this protocol can also be used to treat a wide variety of other health problems like carbon monoxide poising and diabetic foot ulcers?
If you plan on using the HBOT device for yourself or loved one, note that some claims of what it can do are unproven yet. For instance, hyperbaric oxygen therapy devices haven’t been proven yet to cure cancer, Alzheimer’s disease or autism.
The U.S food and drug administration even recommends you always check with your health care provider before opting for HBOT. This is to ensure you’re using the appropriate and suitable care. Does your healthcare provider recommends HBOT? If yes then FDA suggests you go to a hospital or facility that has undergone through inspection and has been properly accredited by the Undersea and Hyperbaric Medical Society (UHMS).
Let’s have a review on FDA’s actual stance on HBOT so as not to give customers the wrong impression.
HBOT and FDA’s Role and Stance On It

It is essential to note that the safety and effectiveness of HBOT hasn’t been established by the FDA for certain conditions, however, some individuals have used hyperbaric oxygen therapy as a viable treatment option for each. FDA reports that 27 complains have been submitted from health professionals and customers within the span of 3years. These reports are totally in regard of treatment centres promoting the use of hyperbaric oxygen therapy for treating conditions not approved by the agency.
HBOT basically involves the breaking of 100% pure oxygen in a special enclosure known as a hyperbaric chamber. The air pressure in the chamber enables the lungs to collect more oxygen which helps in transporting more oxygen to the tissues that are in need of it to aid the body in healing and fighting off certain infections. But for your information, too much oxygen can cause severe damage to the body. FDA thereby regulate both the oxygen used during HBOT, and also the OXYHELP hyperbaric oxygen chambers.
In other words, the human body needs a sufficient amount of oxygen to function well. According to FDA, HBOT boosts the level of oxygen that’s dissolved in your blood. This increase in the blood oxygen may help improve oxygen delivery to vital tissue functions to help against infection and also minimize injury.
How are Hyperbaric Chambers Cleared by FDA?
FDA clearance of a medical device basically involves a determination that the device has got some effects and use, and is safe as another legally U.S-marketed device of that type. In July 2024, FDA cleared hyperbaric chambers for the following disorders;
- anemia (austere anemia when blood transfusions won’t help)
- air and gas bubbles in blood vessels
- burns
- carbon monoxide poisoning
- gas gangrene
- crush injury
- loss of vision wounds (non-healing, diabetic foot ulcers)
Currently, HBOT is being studied for conditions including COVID-19. However, it hasn’t been cleared or authorized for the treatment of it or any other condition that aren’t highlighted in this article.
Are There any Risk of HBOT?
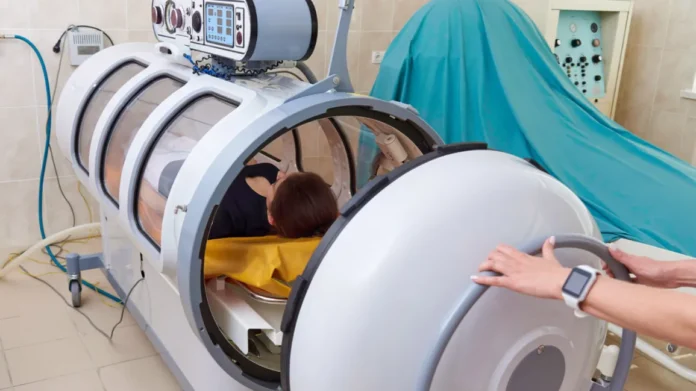
According to FDA, when HBOT chambers are used for conditions that has been cleared and approved, HBOT is considered safe, and severe complications are rare. But, due to the increase in pressure and oxygen concentration, during HBOT, possible risks include;
- Temporary vision change
- Ear and sinus pain
- Lung collapse
- Middle ear injuries
When there’s high concentration of oxygen, it also poses a risk of fire. This is why FDA advises treatment should be carried out at an accredited facility. Those conditions which hasn’t been cleared by FDA are referred to as off-label conditions. Below is a list of all off-label HBOT uses that hasn’t been cleared by FDA.
- Asthma
- Alzheimer’s diseases
- Bell’s palsy
- HIV/AIDS
- Cerebral palsy
- Brain injury
- Depression
- Hepatitis
- Migraine
- Heart disease
- Parkinson’s disease
- Multiple sclerosis
- Heart disease
- Sports injury
What are Hyperbaric Devices?
During HBOT, the user is placed in a special chamber where oxygen in high pressure is been pumped into it. These chambers comes in either Monoplace (for one user) or Multiplace (more than one user).
Aside from the hyperbaric oxygen chambers, zippered chambers are also hyperbaric devices notable for treating altitude sickness. They provide pressure but aren’t attached to oxygen tanks. Currently, these bags haven’t been cleared for use by FDA with oxygen tanks or oxygen concentrators. However, there’s been situations where people use them to create homemade HBOT devices, resulting to fire and suffocation.
When talking about hyperbaric chambers, the OXYHELP hyperbaric chambers makes a good option.
What are OXYHELP Chambers?

The OXYHELP hyperbaric oxygen chambers are designed for use basically at home, spa or even wellness centres. It comprises of both the OXYLIFE I (Monoplace hyperbaric chambers) and OXYLIFE C (Multiplace Hyperbaric Chambers). Note that, these products are declared non-medical, commercial devices basically for wellness and relaxation purposes. Unlike other chambers, the temperature inside OXYHELP hyperbaric chambers depends on the user’s body heat, and the compressor usage time.
Are OXYHELP Hyperbaric Chambers FDA Approved?
No, OXYHELP hyperbaric chambers are not yet approved by FDA in the United States. As earlier stated, they are non-medical devices that helps to increase performance for sports, and boost energy for everyday needs. These chambers also work well in injury recoveries, anti-aging beauty.
Note: Are you experiencing any health or safety issues related to HBOT? Then you can report these cases to Med watch – an FDA safety information and adverse event reporting program.