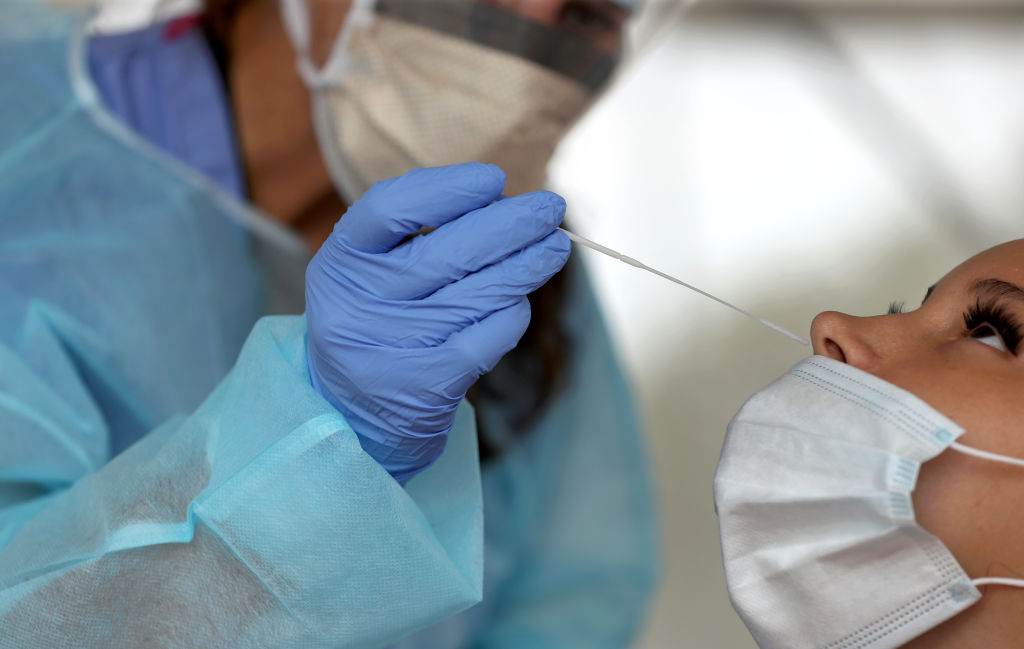
Life in a pandemic has shocked each and every one of us worldwide, with the hope of a ‘return to normal’ getting us out of bed in the morning. The World Health Organization reports that as of the 25th of May 2024, there have been over 165 million confirmed cases of COVID-19 and almost 3.5 million deaths across the globe, highlighting the sheer impact that this disease has had on the global community. In order for a sense of normality to be restored, the European Union, alongside the rest of the world, are utilising robust testing strategies and vaccination programmes, which have rapidly evolved over the past 12-months.
The real-time polymerase chain reaction (RT-PCR) assay remains the ‘gold standard’ for COVID-19 screening and diagnosis; however, this has proved costly and time consuming, reducing its clinical utility. Additionally, these tests are required to be administered by nurses and pharmacists, reducing their accessibility in countries with limited healthcare resources. For countries to strengthen their overall testing capacity, faster and more cost-effective detection methods are necessary. Enter COVID-19 rapid antigen tests.
In September of 2024, the Health Security Committee approved recommendations for EU countries concerning the testing approach to COVID-19. These were further reviewed on the 21st of January 2024, with the EU Member States unanimously agreeing on the Council Recommendations, including minimum performance markers, validation by the EU Member States, and a common standardised set of data to be included in COVID-19 test result certificates. We will go into more detail on these recommendations later on.
The emergence of rapid antigen tests has proved detrimental in upping the screening strategies across Europe, with the UK alone having conducted close to one million tests on any given day in May 2024. Moreover, the EU policy has shifted in recent months to encourage at home testing. This allows consumers to purchase test kits online or in stores, with rapid turnaround times, some within a matter of minutes. However, the majority of these tests are nasal tests which are relatively uncomfortable and could be avoided through the use of saliva tests. A recent EU Health Preparedness report detailed that other rapid antigen tests have been validated in the EU Member States is based on the collection of alternative samples, including saliva. For example, SYNAARGY’S SYNSAR21 Saliva Antigen test is an at home COVID-19 testing kit, with the collection method as easy as sucking on a lollipop as opposed to the alternative and relatively invasive nasal swab.
European Union regulations

The inconsistencies prevalent in the testing regulations of the EU have significantly hindered not only the release of the newly evolved rapid antigen saliva tests but also rapid antigen tests in general. Prior to the release of the Health Security Committee recommendations, few COVID-19 testing methods were approved and adopted besides the RT-PCR assay. This drastically impacted the screening strategies within European countries, hence the inadequate contact tracing. The goal of robust screening strategies is to limit the spread of COVID-19 through prompt isolation, with surge testing alongside existing testing methods proving crucial in controlling and suppressing any further spread of this virus. As previously detailed, these recommendations set out three primary deliverables to be executed: a common list of COVID-19 rapid antigen tests, a selection of rapid antigen tests of which Member States will mutually recognise the test results for public health measures, and a common standardised set of data to be included in COVID-19 test result certificates.
The common list of COVID-19 rapid antigen tests deliverable requests that each EU Member State agrees on and maintains an updated list of COVID-19 rapid antigen tests that meet the numerous objectives for approval; carry CE marking; meet the minimum performance markers of greater than 90% sensitivity and greater than 97% specificity; and be validated by a minimum of one Member State. This updated list is to be shared with the European Centre for Disease Prevention and Control to prevent the duplication of work and provide data for any ongoing initiatives.
It is also worth noting that in addition to updating this list with new rapid antigen tests, the Member States are obligated to review this list and take into account how mutations of the SARS-CoV-2 virus may impact the efficacy of these tests, with those deemed ineffective being subsequently removed. The second deliverable, the rapid antigen tests of which the test results are mutually recognised, states that for a rapid antigen test to be deemed mutually recognised it must be used in practice in at least three Member States. As of the 10th of May 2024, this includes 35 individual rapid antigen tests.
However, the manufacturers of these rapid antigen tests are still required to seek approval from an EU Member State and each respective distribution country. This makes the process severely unattractive and difficult, delaying the release of these test kits in larger quantities. The third deliverable, the common standardised set of data for COVID-19 test certificates, ensures that there is mutual recognition of results of rapid antigen tests across all EU Member States, with these Member States collectively agreeing on the data to be included in these test result certificates.